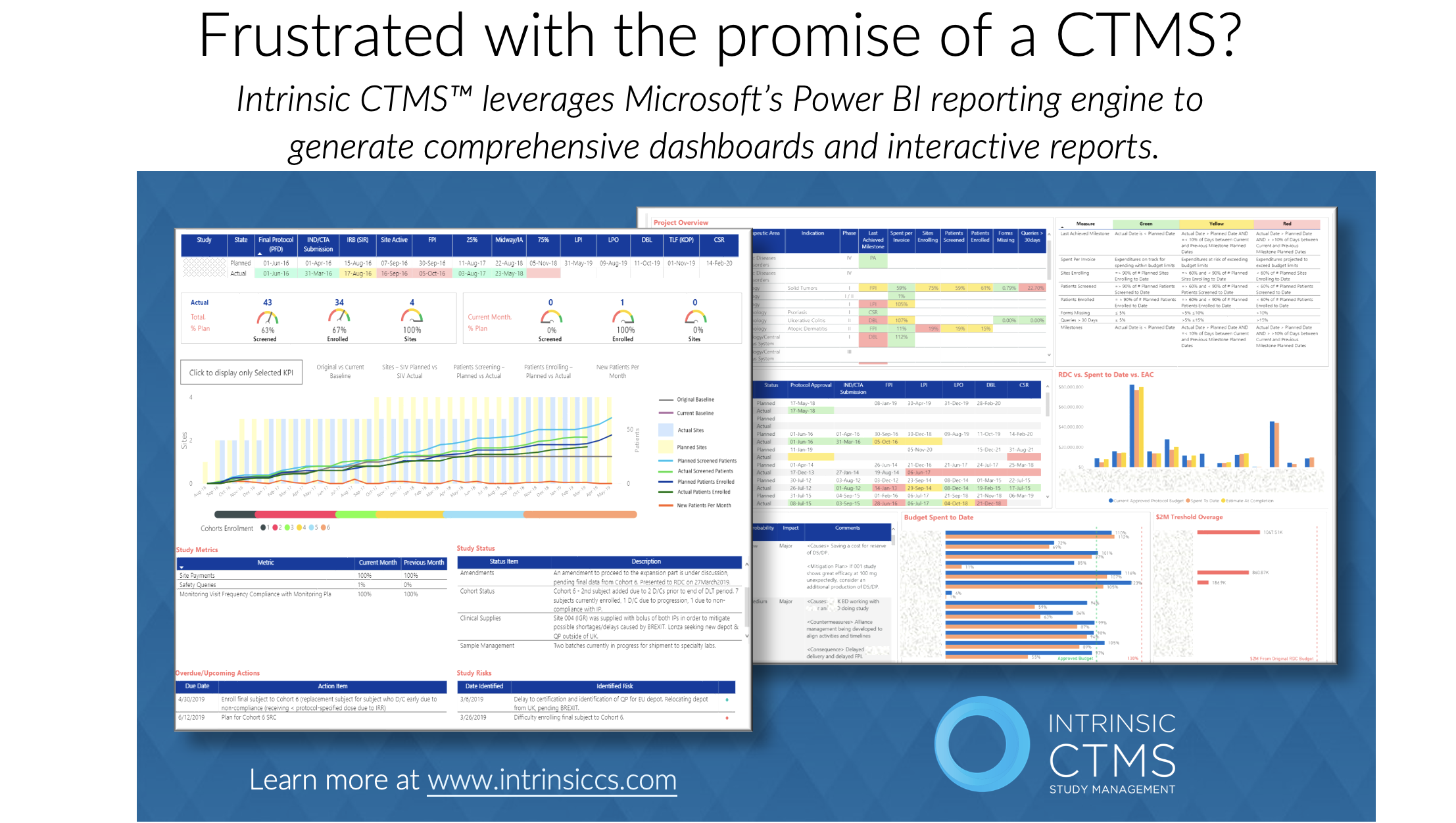
In the realm of clinical trials, data holds immense value. Timely access to accurate and comprehensive data is crucial for making informed decisions that can drive trial success. Clinical Trial Management Systems (CTMS) play a vital role in facilitating real-time data access, enabling researchers and stakeholders to make data-driven decisions. In this article, we will explore how Intrinsic’s CTMS product offering empowers data-driven decision making by providing real-time access to trial data.
1. Central Data Repository:
CTMS serves as a central repository for storing and managing all trial-related data. It consolidates various sources of data, including participant information, study protocols, monitoring reports, and safety data, into a single platform. By centralizing data management, CTMS ensures that stakeholders have a comprehensive view of trial data, eliminating the need to search through multiple systems or databases. This centralization organizes information and also enables efficient data access.
2. Real-Time Data:
One of the significant advantages of CTMS is its ability to provide real-time data updates which eliminates stale information associated with manual processes. As data is captured within the system, it becomes instantly available to authorized users. This real-time data access allows researchers and stakeholders to monitor trial progress, track participant enrollment, and review data quality metrics as they unfold. By having up-to-date information at their fingertips, decision makers can respond promptly to challenges, identify trends, and make timely adjustments to ensure trial success.
3. Data Quality Monitoring:
CTMS enables continuous monitoring of data quality in real-time. The system provides tools and functionalities for assessing data completeness, accuracy, and integrity. Researchers can set up data quality checks and alerts within CTMS to identify potential discrepancies or outliers. Real-time data quality allows for monitoring and oversight for immediate action to address any issues, ensuring the integrity and reliability of trial data. By maintaining high data quality standards, CTMS empowers data-driven decision making based on accurate and trustworthy information.
4. Customizable Reporting and Analytics:
CTMS offers robust reporting and analytics capabilities, allowing researchers and stakeholders to generate customized reports and easily analyze trial data. The system provides pre-defined metrics and visualization tools that enable researchers to gain insights into study progress, participant demographics, enrollment rates, and key performance indicators. Customizable reports and analytics empower decision makers to track trial milestones, identify areas for improvement, and make data-driven decisions based on comprehensive and up-to-date information. The Intrinsic CTMS software contains detailed reports that are available out of the box. Intrinsic also offers a team of experts that will work hand in hand with you to create customized reports based on your unique business needs.
5. Remote Data Access:
In an era of remote work and decentralized trial operations, CTMS enables remote data access, ensuring that stakeholders can retrieve trial data from anywhere, at any time. Researchers and stakeholders can access the CTMS platform securely via web-based interfaces, eliminating the need for physical access to specific locations or systems. This remote data access facilitates efficient decision making by providing stakeholders with real-time access to critical trial information, regardless of their geographical location.
6. Data-Driven Risk Management:
CTMS supports data-driven risk management by providing real-time access to key risk indicators and facilitating risk assessment. Researchers can monitor and analyze data within CTMS to identify potential risks, such as protocol deviations, adverse events, or data anomalies. Having access to risk data enables proactive risk mitigation and the implementation of risk-based monitoring strategies. By leveraging data-driven risk management, CTMS enhances trial quality and supports efficient decision making to ensure participant safety and trial success.
7. Enhanced Collaboration and Communication:
CTMS fosters collaboration and communication across trial stakeholders, making teams work together more efficiently. The system provides features such as messaging systems and notifications that enable stakeholders to exchange information seamlessly. Real-time data access within CTMS promotes
transparency and ensures that all stakeholders are aligned and well-informed. This enhanced communication enables team members to drive better study results.
The key take away is that CTMS plays a vital role in empowering data-driven decision making in clinical trials. With CTMS, researchers can harness the power of data to drive trial success, improve operational efficiency, and ultimately bring safe and effective treatments to patients more efficiently. To learn more about the Intrinsic CTMS offering please request a demo today!
Schedule Demo Here: https://intrinsiccs.com/ctms/