In the realm of clinical trials, data holds immense value. Timely access to accurate and comprehensive data is crucial for making informed decisions that can drive trial success. Clinical Trial Management Systems (CTMS) play a vital role in facilitating real-time data access, enabling researchers and stakeholders to make data-driven decisions. In this article, we will […]

A Clinical Trial Management System (CTMS) can streamline and optimize your organization’s clinical trial operations. A well-implemented CTMS will assist in planning, tracking, and managing various aspects of clinical trials. Your organization can ensure a successful and effective CTMS implementation through careful planning and adherence to best practices. Define Clear Objectives Defining clear objectives before […]

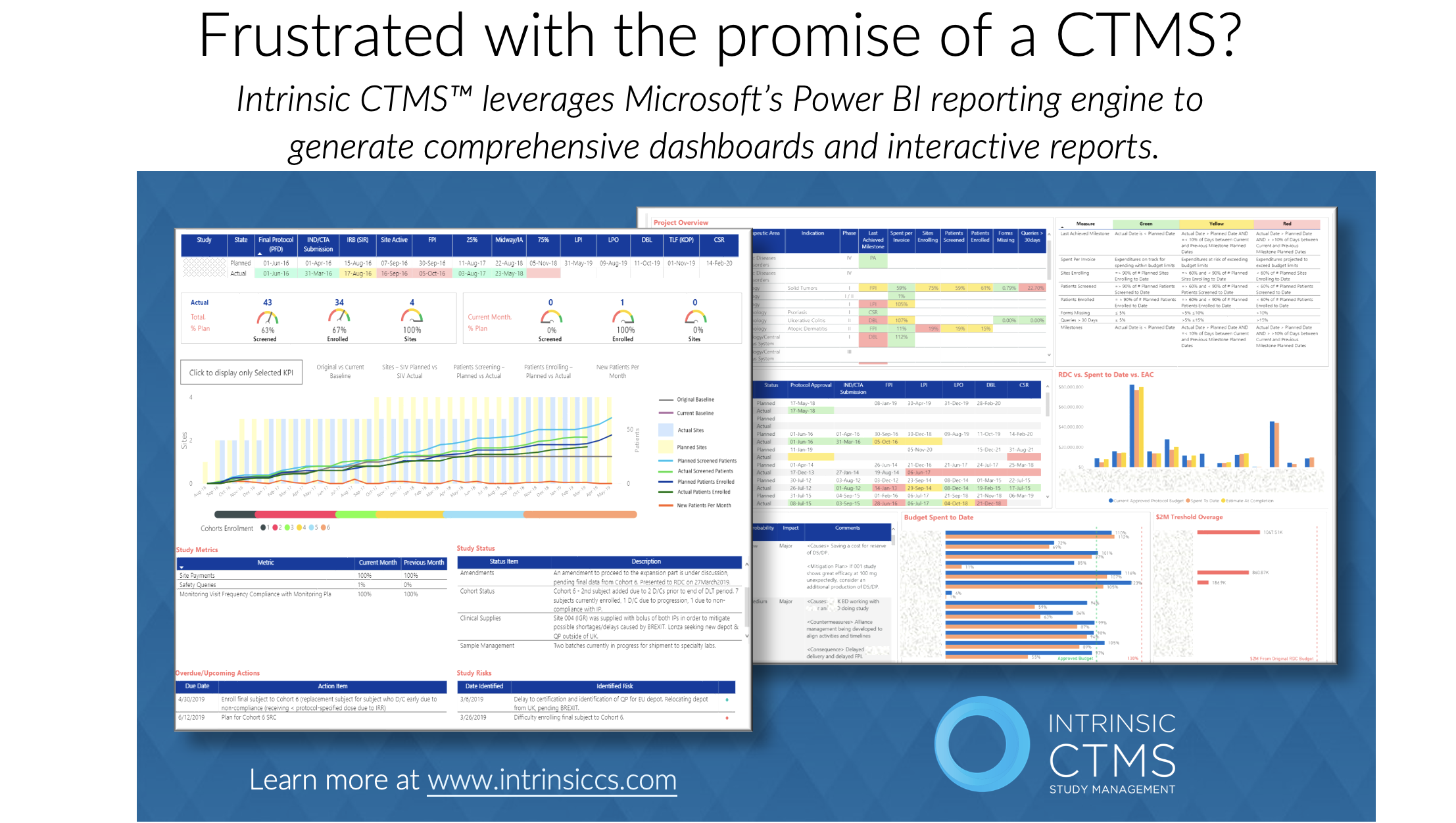
Clinical trials are pivotal for medical advancements, offering new treatment possibilities and better patient outcomes. However, their complexity, which involves various stakeholders, unique processes, and extensive data, can be daunting. To streamline operations, ensure regulatory compliance, and achieve successful outcomes, organizations require a robust Clinical Trial Management Software (CTMS) solution. Intrinsic CTMS is designed to […]

Data prioritization is essential for business success as it sheds light on customer needs, revenue generation, and trend analysis, especially critical in managing clinical trial data due to its involvement in life-saving treatments and patient well-being. Even a little effort in turning data into insights makes a big difference, improving understanding and guiding decisions. This […]

In the ever-evolving landscape of clinical trials, the need for a Clinical Trial Management System (CTMS) that is configurable, flexible, and scalable cannot be overstated. In this era of precision medicine and fast-paced research, organizations require a CTMS solution that can adapt to their unique protocols, processes, and changing requirements. But what sets apart an […]

Pharmaceutical companies have been operating in the cloud for over a decade. For clinical trials, EDC (Electronic Data Capture) was the first of the eClinical systems to move into the cloud. Cloud-based CTMS (Clinical Trial Management Systems) have had a slower cloud adoption, but in the last several years, pharmaceutical companies have been pushing for […]

Recently I wrote a blog about the steps required to successfully identify and select a clinical systems vendor that will truly deliver against your needs. Here is the recap of that post: Write a business case Collect detailed requirements Write a clear RFI Quickly narrow down your list of candidates Host some dog & pony […]

The systems used in clinical R&D (or commercial, or HR, or finance…), like everything else in the world (people included), eventually grow old and stop working the way we’d like them to. Fortunately, these systems can be replaced – though not necessarily any more cheaply or easily than your Uncle Irwin’s hip. Done the right […]

In today’s environment, it is almost certain that the pharmaceutical industry will continue the downsizing of drug development operations. In doing this, they will place an ever-greater reliance on strategic CRO alliances and collaborative technologies. As sponsors increase their reliance on CROs, the need for better collaboration and more transparency will also increase. Data Collection In […]

At Intrinsic, we’ve been implementing Clinical Trial Management Systems (CTMSs) and have been part of the eClinical evolution since Part 11 was drafted back in 1997. During that time, we’ve had to answer a lot of questions, starting with, ‘Do I have to validate my CTMS?’. Over the years that question was replaced with, ‘Do […]









