
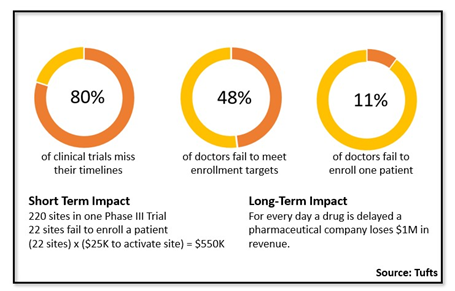
If there was one problem you could fix with clinical trials, what would it be? Would it be better supply chain? Better clinical trial software? More qualified clinical trial personnel, such as clinical project managers? Sure, all of these are serious challenges we face day in and day out, but many clinical team members would argue the most important problem to solve is patient enrollment for clinical trials. For decades, we as an industry have tried to fix this patient enrollment problem. More recently, we have tried to fix this problem with leveraging internal investigator data and purchasing external data, such as claims data. All of this is certainly helping, yet we still have the same old problem. According to Tufts, eighty percent of clinical trials still miss their clinical trial enrollment timelines and forty eight percent fail to meet clinical trial enrollment targets. In addition, eleven percent fail to enroll one patient for a clinical trial. For a phase three trial, the site activation costs for failed investigators can easily total $500k. And this does not even consider the costs of trying to get the struggling clinical trial sites back on track, adding rescue sites, or finding new clinical trial sites in a hurry. The long-term impact on revenue is staggering and often a gray area. Let’s just say this is millions upon millions of dollars.
We continually utilize data to estimate where patients live in order to select the best sites. There have been many large companies and startups focused on patient populations and matching patients to clinical trial inclusion/exclusion criteria, leveraging a website or clinical trial artificial intelligence to find where the ‘best’ patients are. IBM Watson is an example where IBM Clinical Trial Matching enables clinicians to more easily and quickly find a list of clinical trials for an eligible patient, and in the clinical trial office, find patients that are potentially eligible for any of the site’s trials. IBM claims they can help increase clinical trial enrollment targets and opportunities to offer patients the option of a clinical trial for treatment.
Now, am I suggesting you purchase the IBM Clinical Trial Matching software? No, but I do believe data in some way should be utilized for clinical trial patient enrollment. In the end, clinical trials are always about people. People in the pharmaceutical industry do the daily grind because of some deeper meaning. And for those involved in clinical trials, it’s about the patients. We all have been affected directly or indirectly by some disease. Our drive to help patients is why relationships are so important. We are in a people business.
So, while data is important, relationships are more important. And it’s not just the patients, but also the clinical trial investigators and all site personnel, not to mention the broader universe of clinical trial site personnel, such as vendors.
This seems like an obvious statement, yet many pharmaceutical and biotech companies do not focus enough on site relationship management (SRM). They do not have centralized data on investigator performance, investigator relationships, site communications, which investigators participated in previous trials, etc. Data on clinical trial site personnel and clinical trial site performance is all over the place! Think about it. How much data is spread over thousands of email addresses within one pharma company? Thousands of emails with interactions that tell a story about a relationship. Now some people may argue it’s the face-to-face interactions that matter. I agree, but how often does that happen? Some may argue it’s the phone call discussions that matter. I agree, but again, how often do you have a phone call these days? I would like to see a statistic about how much of a relationship with site personnel takes place via email – 50%? 75%? It’s possible. So why is this information not centralized? And more importantly, why is it not used to foster better clinical trial relationships?
Thinking about relationships, have you ever received an email that has nothing to do with you? Let’s say a vendor in the pharmaceutical industry sends you an email asking if you need consultants for market access. You are in clinical, not in commercial…delete. Now, let’s say your site only conducts oncology trials and you work in France. You receive an email from a pharmaceutical company about clinical trial feasibility on a CNS trial in the United States…delete or even worse unsubscribe, not knowing that the pharmaceutical company also conducts clinical trials in CNS. The pharmaceutical company sent a blast to sites who should not be on that distribution list. Why? Because the data about the sites and site personnel is not centralized, and certainly not utilized in any helpful way. But its not about the email…it’s about the relationship. This is where pharmaceutical and biotech companies need to focus. We live in a digital world and pharmaceutical and biotech companies need to produce quality content to keep and strengthen relationships. And an email is digital content.
So, back to the original question – if you could fix one problem in clinical trials what would you fix? Have you thought about the benefits of fixing relationships with clinical trial site personnel, vendor personnel, CRO personnel, etc.? There is world class software that manages relationships, with the sole purpose of improving them. Companies should consider using a CRM system to manage and improve relationships. With better relationships pharma companies may receive more accurate clinical trial enrollment projections, quicker responses to clinical trial feasibility, more engagement from clinical trial sites throughout the clinical trial, etc. Oh, and what does CRM stand for, you ask? Customer Relationship Management! But we don’t use CRM software in clinical trials. It’s like saying companies don’t use accounting software to manage their debits and credits. Of course, they do. And one other thing, email or Microsoft Outlook is not a CRM system. In fact, it’s the opposite of what a CRM system is all about, because the information is decentralized. This leads to random communication and frustration on the part of our contacts, instead of focused, thoughtful communication.
Having discussed how we communicate in clinical trials and how relationships are currently managed from a digital perspective, let’s discuss an ideal state on how clinical trial relationships should be managed.
Let’s review five use cases that occur in every clinical trial.
- Everyday email communication
- Which investigators should we consider for a clinical trial?
- Clinical trial site feasibility
- Study newsletters and protocol amendments
- Satisfaction surveys
Everyday Email Communication
The question about everyday clinical trial email communication with clinical trial site personnel and other vendors is, is the information important? In other words, I may send an email to a site coordinator saying, we did not receive a 1572 form, or enrollment is behind target, try these five things. Is this important? Is it important to track all of these clinical trial site communications centrally in a system? Of course, it is. Site relationship management (SRM) and investigator relationship management (IRM) is important. The purpose of a CRM system is to track discussions with site contacts and foster relationships. If this information is stored centrally then your colleagues can see what has been communicated to clinical trial sites. This prevents clinical trial sites from being contacted over and over again and asked for the same information, which drives the very sites you want to partner with crazy. In addition, clinical team members now have a wealth of data to analyze to find common challenges. Or, if you have a phone call with the site coordinator to share what has worked well for other sites regarding clinical trial patient enrollment, you can type the notes into your CRM system for others to see. If all that sounds like a big pain to you, fear not. The great thing about Microsoft Dynamics CRM is its native integration with Outlook and other Office 365 applications. The clinical team member creating the email just clicks a button on the email in Outlook and the email automatically gets stored in CRM Dynamics under that contact, and all the responses to the email get tracked as well. And if CRM Dynamics is setup properly, the communication can be posted to a clinical site page where all discussions (emails, phone calls, etc.) are automatically posted. That way team members can also answer the question of when was the last time we communicated with this clinical trial site, what was communicated, etc. The picture below describes the old process versus the new, ideal process for everyday communication.
The new, ideal process only requires one more step from a clinical team member, clicking a button to track the email in CRM. That’s it. We are not asking clinical team members to login to a new system. We are not breaking their flow of work. The clinical team members stay in Outlook and just click a button! That simple. Now, information is stored centrally leading to better site relationships.
Which Investigators Should We Consider for a Clinical Trial (Leads)?
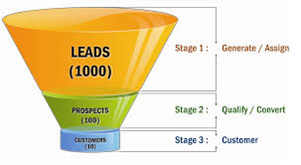
The second use case is for a new clinical trial and answering the question of which investigators should be initially considered. Again, some companies may have the relevant site information in a variety of clinical systems, such as CTMS, EDC, or maybe an investigator database. Others have this information in Excel spreadsheets that act as a CTMS or investigator database. And others have this information spread over many CROs. In all these scenarios, why is data scattered? This is not investigator relationship management (IRM) or site relationship management (SRM). All clinical team members should have access to a list of investigators, their address (country), email address, clinical trial experience, therapeutic area experience, licenses, past clinical trial performance, etc. If all this data is centralized, clinical team members simply filter by these parameters to get their initial cut of which investigators to consider for a trial. Conducting a trial in the United States? Great, filter on United States to target those sites. Need sites with oncology experience? Filter on oncology. You get the point, and what was just described is no different than a classic marketing funnel. Start with 1000 leads, qualify them and narrow the list to 100 prospects and then convert the best prospects to 10 customers. This funnel type approach is efficient and how clinical team members should consider selecting investigators.
Site Feasibility (Prospects)
Now, let’s consider the next use case for improving site relationships, clinical trial site feasibility. There are many types of feasibility, such as protocol feasibility, country feasibility, site feasibility, etc. If you’re doing site feasibility, do you utilize Survey Monkey, Word documents or similar tools for clinical trial feasibility? How much time is wasted collecting all this data, which typically gets tossed after the study is over? Feasibility should be completed right in your CRM system. Why? Because clinical team members need to send the feasibility to people who are customers. The customers have an email address, which should be a data point on their customer contact in a CRM system. Each site’s email address can be part of a site email list and study email list that is easily reusable by all clinical team members. That communication (feasibility) should be stored centrally with all day-to-day communication for all sites for all trials. This is site relationship management (SRM) or investigator relationship management (IRM).
Study Newsletters and Protocol Amendments
Another use case to consider for site relationship management (SRM) or investigator relationship management (IRM) are emails sent from a sponsor to all sites, such as a study newsletter, protocol amendments, etc. Do you currently type the names in an email? Some companies do and others use Outlook mailing lists. Do you recreate the message each month? Do you know if a new site team member received the email? Do you know who opened the email? Do you know if the email bounced and if the email address was corrected, was it stored centrally so it did not bounce again? Do you know who responded to the email? This is all part of site relationship management (SRM) or investigator relationship management (IRM). It’s crazy if you think about the micro productivity issues we don’t even question, let alone the lack of stored, centralized data that could be used for analytics for a study, and, this is equally important, across all studies. An email to all sites should be simple as, select an email template (reuse), type the information or copy information from another communication (reuse), select an email list (reuse) and send the communication. The amount of time savings adds up and the change in business process is not a big one.
Satisfaction Surveys
Ever wonder how well you are doing as a sponsor working with a site? Ever wonder if your vendors are frustrated working with you as a sponsor and what you can do to improve. Guess what? Most pharma companies are concerned about relationships with sites, and feedback leads to improved relationships. It’s about contacts. Having a central place where common survey questions are dragged and dropped onto a canvas that acts as a survey can make your life a lot easier; select an email list and send. If you need reports on how many people opened the email, how many responded, etc., then analytics on the responses are automatically generated with a CRM system.
Contacts Are Key to Relationships
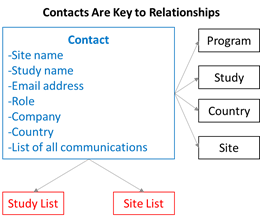
Several use cases were discussed, and every use case is about data points that describe a contact and the relationship of that contact to other items. For example, a contact is associated with a site. The site is associated with a country. The country is associated with a study. Connecting all these attributes is what makes this process so much better. It’s the essence of what a CRM system does. And if the CRM system is configured properly, all these use cases should be integrated and seamless in one tool. Why buy a one-off CTMS system? Why buy a one-off feasibility tool? Why buy a one-off site relationship management tool (SRM) or investigator relationship management system (IRM)? Why buy an investigator database? This is all the same stuff with the fundamental piece of information being a contact who becomes a customer. And those customers need a place to live. Think about Facebook and LinkedIn. Could you imagine living without these systems! Insane to think about it. How did we live without these apps? They basically allow us to live our busy lives and still have relationships in our digital world. So, these clinical trial contacts that become customers, why are they not in some version of a social network, such as Facebook and LinkedIn? Why are the customers not in a CUSTOMER relationship management system???? What we are saying is we have not enabled our clinical customers to have a better relationship with the sponsors. It’s crazy and so obvious once you begin thinking about it. Consider the picture below. If a contact has certain data attributes, such as a site name and an email address, then the contact can be added to a site list to receive communication. The examples are endless.
The most advanced pharma companies have a CTMS, an investigator database, a feasibility tool, etc. In my mind it’s all the same thing, yet it needs to be thought about differently. All these tools exist because ten to twenty years ago, when vendors created these tools, a good CRM system did not exist. Now, we use point solutions for point processes. Just purchase a tool which does all this seamlessly with one important consideration, the contact should be the central focus of the system. Let, a CRM system replace all these systems. With the contact as the focal point, relationships develop and strengthen.
Consider the history of investigator relationship management (IRM) and site relationship management (SRM). Around 2008, there was a movement toward investigator relationship management (IRM) and site relationship management (SRM). https://www.reliasmedia.com/articles/12938-site-relationship-management-sponsors-help-sites-be-more-productive
- Many people, such as Beth Harper, argued the term site relationship management (SRM) is more appropriate in her article “Site relationship management (SRM): Sponsors help sites be more productive”.
- During the same period, Sanofi Aventis’ program began at square one. They surveyed more than 5,000 sites to find out where to focus their efforts. The sites were clear in what they wanted: more effective communication between the site and sponsor. More effective communication!
- The focus at Eli Lilly hinged upon determining the criteria for identifying high-performance sites. Eli Lilly calls these sites Portfolio Sites and plans to forge intense relationships with them. Again, the focus is on RELATIONSHIPS!
As time went on, companies found some solutions but since 2008 we dramatically moved into a digital world, which was facilitated in our personnel lives with Facebook and LinkedIn. This digital world has yet to move into our work world in clinical trials. Don’t believe me? Google “site relationship management”, “SRM”, or “clinical trial”. Two pages of results!! That’s it. Now change the Google search so the time is restricted from January 2018 to today, roughly three years. What does Google provide for a search result? “It looks like there aren’t any great matches for your search”! Google can’t find anything! It’s crazy. CRM is not new. We just have not discovered how to digitize what we currently do in a way that has customer relationships as its focal point.
Taking this a step further, the analytics we could do with a CRM are astounding. Start with the basics. Out of the box, Microsoft CRM is integrated with Microsoft Power BI. For those not familiar with Power BI, Gartner consistently ranks the top three reporting tools as Tableau, Qlik and Power BI where Power BI in recent years has pulled out in front of the other two. With this connection between Power BI and Microsoft CRM Dynamics, the whole data model from CRM is exposed in Power BI! It’s easy to create any kind of report. And because you implemented CRM across the whole clinical trial process with many different use cases, you have access to all kinds of analytics.
Taking this a step even further, consider artificial intelligence (AI). Think about the hundreds of thousands of interactions for just one trial, in one database. It’s a gold mine of information to use for many different use cases and all based on relationships. You might say, yeah this sounds farfetched. I get it. But remember that first time Facebook or LinkedIn suggested people you might know and want to connect with? I remember. It freaked me out. How does this application know who I know, yet it was spot on with its recommendations on who to connect with? The algorithms worked because the data knew connections…it knew relationships. In the future, and not too far off, artificial intelligence will be using this data to improve relationships, and that is the problem we need to solve. If we as clinical trial workers develop more relationships, better relationships, more fluid relationships and relationships that allow us to connect and work more easily, then the patient enrollment problem will be solved.